Vaccine effectiveness and safety at heart of FDA only granting EUA for COVID booster
Waning immunity from vaccines was at the center of the debate last Friday among members of a Food and Drug Administration (FDA) panel. They were asked to consider approving a third booster shot of the Pfizer vaccine.
On the same day, the Centers for Disease Control (CDC) reported the Pfizer mRNA vaccine delivers less long-term protection from hospitalization than the Moderna vaccine. It remains higher than the Johnson and Johnson, single shot vaccine. All vaccines offer much greater protection from hospitalization than no vaccine.
The FDA panel rejected Pfizer’s request to grant general approval for all people to receive a “booster” third injection of their vaccination. Instead it provided EUA authorization for use in at-risk older Americans and those with serious comorbidities. Dr. Steven Pergam of the University of Washington’s Fred Hutchinson Cancer Center was one of those voting against the general authorization.
These developments only add to some people’s concerns regarding our public health agencies and the effectiveness of the rapidly developed COVID-19 vaccinations. Americans began receiving them under an Emergency Use Authorization (EUA) from the FDA, which only recently granted normal approval for the Pfizer vaccine alone. The other two continue to be authorized under the EUA.
At the FDA proceedings, Pfizer was seeking approval for what President Joe Biden had promised citizens on Aug. 18 — a booster shot for every American.
An eight hour virtual meeting was held that included an hour of an open comment public hearing. Questions were raised about the lack of data and the small sample size in Pfizer’s booster study. Others questioned the efficacy of the vaccines in general due to the rapidly diminishing effectiveness.
A great deal of information and discussion centered around Israel, which almost exclusively used the Pfizer-BioNTech vaccination. They experienced a “fourth wave” of COVID-19 in July and immediately began administering a third booster shot of the Pfizer vaccine.
During questioning, Dr. Sharon Alroy-Preis of the Israel Ministry of Health revealed the following.
“What we saw prior to our booster campaign was that 60 percent of the people in severe and critical condition were immunized, heavily immunized, fully vaccinated,” she said. “And 45 percent of the people who died in the fourth wave were vaccinated.”
But the bigger surprises and concerns were raised over the safety of the vaccines by medical professionals, practicing on the front lines of the battle against the virus. They offered input during the open comment period.
The CDC announcement
On Friday, the CDC reported that while both mRNA vaccines from Pfizer and Moderna reduce the chance of hospitalization from COVID-19 by an initial 91 percent or more of patients, that efficacy wanes after 120 days. The Pfizer vaccine’s level of protection drops to about 77 percent, whereas the Moderna vaccination’s protection remains at 91 percent from an initial 93 percent. The data was collected from 18 states during the period March 11–August 15, 2021.
“Among U.S. adults without immunocompromising conditions, vaccine effectiveness (VE) against COVID-19 hospitalization during March 11–August 15, 2021, was higher for the Moderna vaccine (93 percent) than the Pfizer-BioNTech vaccine (88 percent) and the Janssen vaccine (71 percent).
Although these real-world data suggest some variation in levels of protection by vaccine, all FDA-approved or authorized COVID-19 vaccines provide substantial protection against COVID-19 hospitalization.”
According to one news report, the CDC estimates that 54 percent of Americans have received the Pfizer vaccination.
The report states: “although an immunologic correlation of protection has not been established for COVID-19 vaccines, antibody titers after infection and vaccination have been associated with protection. These real-world data suggest that the 2-dose Moderna and Pfizer-BioNTech mRNA vaccine regimens provide more protection than does the 1-dose Janssen viral vector vaccine regimen. Although the Janssen vaccine had lower observed VE, 1 dose of Janssen vaccine still reduced risk for COVID-19–associated hospitalization by 71 percent.”
The CDC noted important limitations in their report.
“The findings in this report are subject to at least six limitations.
- First, this analysis did not consider children, immunocompromised adults, or VE against COVID-19 that did not result in hospitalization.
- Second, the CIs for the Janssen VE estimates were wide because of the relatively small number of patients who received this vaccine.
- Third, follow-up time was limited to approximately 29 weeks since receipt of full vaccination, and further surveillance of VE over time is warranted.
- Fourth, although VE estimates were adjusted for relevant potential confounders, residual confounding is possible.
- Fifth, product-specific VE by variant, including against Delta variants (B.1.617.2 and AY sublineages), was not evaluated.
- Finally, antibody levels were measured at only a single time point 2–6 weeks after vaccination and changes in antibody response over time as well as cell-mediated immune responses were not assessed.
It should be emphasized that this report was focused on chances of being hospitalized after receiving vaccination and limited to a short period of time following vaccination. The CDC calls for further study of the data.
FDA Hearing on Pfizer booster shot
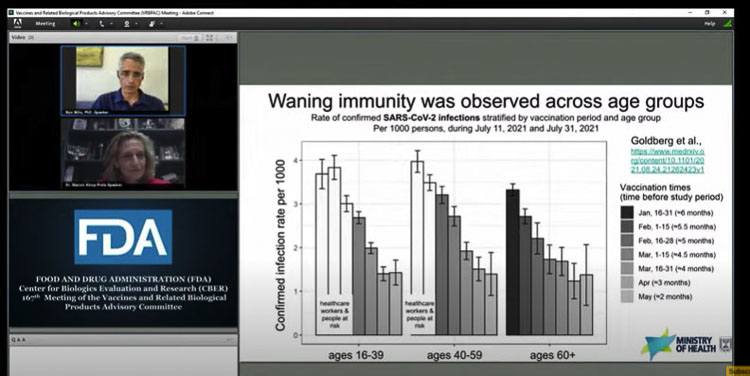
During the FDA hearing, it was noted that Israel is one of the most vaccinated nations in the world and that they have some of the most complete data. It was also reported the Israelis are currently experiencing a “fourth wave” of COVID-19, in spite of its extremely high vaccination rate.
The waning immunity is viewed as not being specific to the Delta variant of COVID-19, but rather the declining immunity the multiple vaccinations offer against the virus. Israel began administering a booster shot, a third dose of the Pfizer vaccination in July.
Bill Gruber of Pfizer noted the protection falls below 70 percent at five months, and it is therefore important to provide a booster shot which will increase antibodies to previous levels.
Questions were raised about the long-term durability of the vaccine — was a booster going to be needed every six months? Members of the panel asked about the increased risk of myocarditis in young people. Some questioned the breakthrough cases and the vaccinated being able to pass on the virus to younger people.
It was during the open comment period that many eye-popping comments and details were revealed.
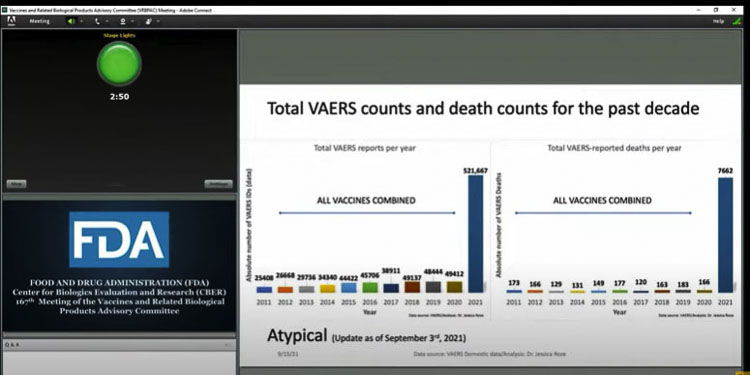
Dr. Jessica Rose showed data from the VAERS (Vaccine Adverse Event Reporting System) site showing over 1,000 percent increase in adverse events due to the COVID vaccinations. This compared annual adverse reports and adverse deaths for all vaccines over the past decade.
Dr. Joseph Fraiman is an Emergency Medicine Physician in New Orleans. He begged the FDA for data and evidence to reduce vaccine hesitancy. “We need larger trials that demonstrate the vaccines reduce hospitalization without finding evidence of serious harm,” he said.
“The fact that we do not have the clinical evidence to show these people they are wrong should terrify us all,” he exclaimed.
Fraiman noted that, independent of education level, the vaccine hesitant he’s met in the ER are more familiar with vaccine studies and more aware of their own COVID risk than the vaccinated. They think their risk of catching COVID and being hospitalized is low. “They’re not wrong,” he said.
A 30-year-old nurse showed him an Oxford Risk Calculator. Her risk of hospitalization is 35 times more likely than death from COVID. She asked if the risk of hospitalization is greater than her risk of harm from the vaccine. He could not honestly answer because the data isn’t there; the sample sizes of the initial studies were too small.
Dr. Fraiman pointed out the initial studies failed to show a risk of myocarditis. We now know the risk is 2 to 3.5 times greater than COVID hospitalization from the virus for young boys.
The former FDA commissioner said the original premise of the vaccine was to reduce deaths and hospitalizations. The initial clinical trials did not find the reduction in death or hospitalization, likely because they were inadequately powered, according to Fraiman “The former commissioner is correct, the initial trials should have been powered to find a reduction in hospitalization,” he said.
Dr. Steve Kirsh spoke to the “elephant in the room.” The vaccines kill more people than it saves, he said. He reported there are “four times as many heart attacks in the Pfizer treatment group — that wasn’t bad luck. VAERS shows it happened 71 times more often than any other vaccine group,” he said.
Kirsh showed a graphic indicating there were two “excess deaths” for every life saved, from VAERS data. He reported there are 411 deaths per million doses. That would translate to about 150,000 people who have died from the vaccine according to Kirsh.
He cited a nursing home study in Canada where about 50 percent of the vaccinated people died, whereas none of the unvaccinated people died.
Kirsh mentioned a new paper posted showing that 1 in 317 boys ages 16-17 will have myocarditis from the vaccine. Furthermore, 1 in 1,000 people aged 18 to 65 are at risk of myocarditis. He projected after a third booster it could be 1 in 25.
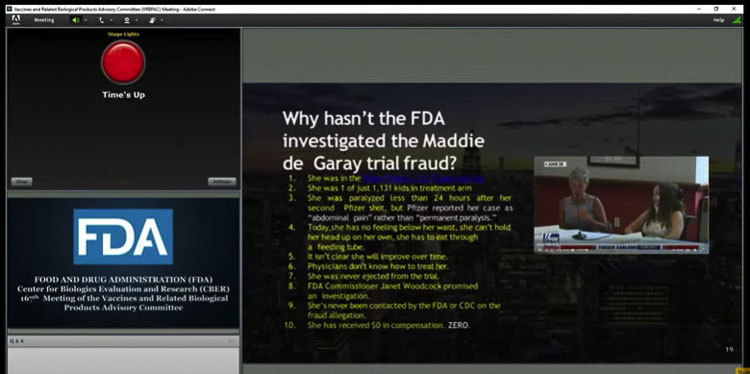
He briefly told the story of 12-year-old Maddie de Garay, who within a day of getting her second dose had significant abdominal problems. A short time later she became paralyzed. Yet Pfizer reported the event as “abdominal discomfort.”
Maddie’s mom Stephanie shared that she cannot digest food, and at one point had convulsions. Stephanie is not anti-vaccine. Yet none of the people at the FDA nor Pfizer have helped Maddie. Stephanie said that Facebook shut down a support group that was trying to help people with vaccine problems.
Dr. David Wiseman said there was an “unclear need, and unclear motivation” for the booster shot. He decried the limited data, and called for large randomized control tests first.
He noted issues about women with menstrual cycle problems and pregnancy concerns. He also cited a need for long term cancer studies and issues related to male fertility.
Wiseman asked the FDA to reverse its policy on both hydroxychloroquine and ivermectin. He noted one study they questioned had been updated (corrected) and showed a 42 percent reduction in COVID-19.
Dr. Peter Doshi cited the CDC’s Jay Butler. “We’re keenly interested in knowing whether or not a third dose may be associated with any higher risk of adverse reactions”. Yet the Pfizer data is not there, according to Doshi. He noted the Pfizer study only enrolled 10,000 “healthy” volunteers for its phase 3 trial. What is the risk to unhealthy individuals?
He closed by asking “what is the FDA doing to ensure the people can speak freely, without fear of reprisal?” He shared that three medical boards, the American Board of Family Medicine, the American Board of Internal Medicine, and the American Board of Pediatrics are all threatening sanctions or discipline against doctors who question the current line of thinking and protocols.
Dr. Michael Carome said Pfizer has failed to provide data and sufficient evidence to show the need for a booster. He said The Lancet just posted a paper saying “current evidence does not support a need for boosting in the general population.”
Dr. Paul Alexander told the panel more longer term studies were needed. He mentioned vaccine accumulation in ovaries and testicles as a concern. He emphasized that COVID-19 is not life threatening for children.
Kim Witczak was listed as an FDA Consumer Representative. “From the beginning of the pandemic, the goal posts keep changing,” she said. “It makes you wonder if the current strategy is working.”
She alleged that Pfizer used data from just over 300 people, only 12 over the age of 65, in their evaluation for the booster. “If the FDA approves this, we will take what we’ve learned on just 300 people and mandate it on hundreds of millions of people,” she said. “This is beyond preposterous.”
“While boosters may be good for business, these mRNA vaccines were never designed to stop transmission or eradicate the virus,” she said. Witczak emphasized the vaccines are not the same as those created to eradicate polio or smallpox.
She closed by asking the FDA to consider the role of natural immunity.
Panel member questions
Dr. Cody Meissner of Tufts Medical Center asked if we should learn from influenza. Shouldn’t we try to match the circulating variant (the delta strain) to the booster? He noted if a different variant becomes dominant, it will likely be derived from the Delta strain.
Dr. Archana Chatterjee, Dean of the Chicago Medical School asked if the panel could explain why there appears to be a difference in breakthrough cases among the vaccinated between the U.S. and Israel. Dr. Sara Oliver of the CDC responded that the definition of “severe disease” is very different in Israel than how we define it in the U.S.
She also noted that Israel achieved high levels of people being vaccinated very quickly, whereas it was slower in the U.S. Oliver noted that Israel almost exclusively used the Pfizer vaccine, whereas in the U.S. we have used three different vaccines.
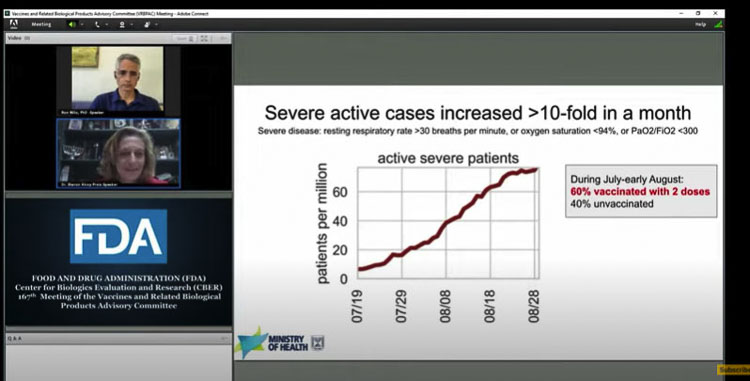
The CDC’s Amanda Cohn asked if Israel has seen an increase in myocarditis, especially in the young. The response was there was a very clear increase after the second dose of the vaccine.
There was a distinct difference in reported myocarditis cases between the U.S. and Israel. VAERS data shows 60 to 70 cases per million doses in the U.S. whereas Israel is reporting 6,000 cases per million doses.
FDA staff responded “We really don’t have enough data yet to know what the risk of myocardial and pericarditis would be in any specific age group.” They further indicated the only way to get specific risk data on a third shot would be “post licensure or post authorization use.”
It was confirmed that in Israel, they have had over 1,000 mortalities in their fourth wave of COVID-19, but 40 percent of those deaths were among the unvaccinated, and 54 percent had received two doses, but not gotten the third booster shot.
“It is clear the double vaccinated play a role in the fourth wave, not only among cases, but in hospitalization and in deaths” the panel was told.
Dr. Hayley Gans of Stanford University Medical Center noted there has already been over 1 million 3rd doses given in the U.S. and she was seeking information on the safety of that 3rd dose. The CDC’s answer was “stay tuned”. They hope to have some information out soon.
One panel member noted that most of those 3rd doses were being administered “off label” (not in medical trials) in the U.S. already.
Dr. Mark Sawyer of UC San Diego shared that the ground rules for the meeting was that the panel was not supposed to consider the data from Israel. Therefore they were limited to the Pfizer trial of 312 individuals, a very small amount of data. He wanted a comparison with other approved vaccines like the meningococcal vaccine. “It strikes me as a little bit low.”
He was told that while some trials have had as low as 300 in the sample size, many more have 1,000 or more.
“What we saw prior to our booster campaign was that 60 percent of the people in severe and critical condition were immunized, heavily immunized, fully vaccinated,” said Dr. Sharon Alroy-Preis of the Israel Ministry of Health. “And 45 percent of the people who died in the fourth wave were vaccinated.”
She shared that it was of huge importance for the booster, not to just reduce the confirmed cases, but actually to save life. It was important for those who are getting the disease and getting into severe or critical conditions.
“The need for an additional vaccination after six months should not be surprising,” said Dr. Peter Marks of the FDA. Nearly half of vaccines require an additional dose.
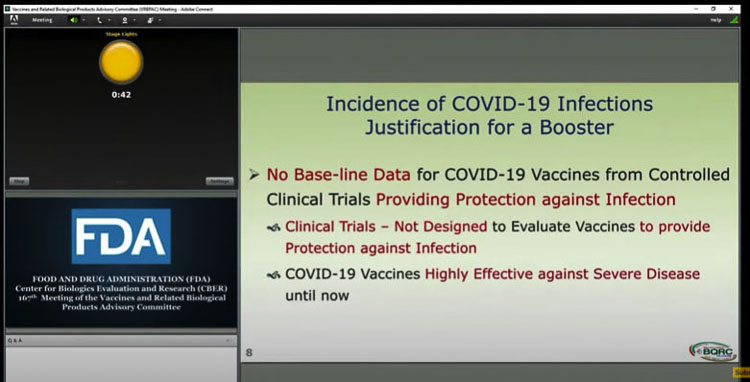
The members of the committee were asked to vote on the following question. “Do the safety and effectiveness data from clinical trial C4591001 support approval of a COMIRNITY booster dose administered at least 6 months after completion of the primary series for use in individuals 16 years of age and older?”
The FDA staff clarified that the panel should “look at the totality of the evidence” when making their decision.
The initial vote was 16 panel members voted against the proposal, and two voted in favor. After which, the panel members explained their concerns.
Dr. Kichael Kurilla of the NIH believes the safety database is inadequate. “We still don’t know the proper interval between doses,” he said. “And I would add to that that we don’t know the proper dose.”
Other panel members echoed that sentiment. Some suggested increasing the length of time between the first and second doses of the vaccine first, believing that might improve the protection of the two-shot series.
Dr. Oveta Fuller was not comfortable with such a small sample size — just 312 in the Pfizer booster study. Dr. Rubin felt there would only be a marginal benefit, given the large number of unvaccinated people. “I don’t think a booster dose is going to significantly contribute to controlling the pandemic,” Meissner said.
“Is it possible to get control of theis pandemic with just two doses,” asked Dr. Offit. He later expanded: “are we talking annual, bi-annual, or tri-annual boosters?”
Several members expressed concerns regarding myocarditis, especially the troubling data on young men. They worried a third dose might make that worse.
Many also cited the need for risk versus benefit data. They acknowledged the elevated risks for senior citizens and the obese or those with serious heart or lung ailments, would benefit from a booster shot. But the benefit for the general population was not known. “The incremental benefit to the younger population has really not been demonstrated at all,” said Kurilla.
“We don’t know that much about risk,” said Rubin. “The truth is a very small number of people under 60 have received the vaccine (booster).”
Following the panel discussion, they then voted to approve a third dose booster shot for those age 65 and older, as well as those deemed at higher risk. They gave this authorization under an EUA, knowing that once more data came in, they could expand approval.
Dr. Pergam emphasized concerns over healthcare workers being exposed due to the continued number of breakthrough cases. He didn’t like offering the booster to everyone, but instead preferred the third shot be targeted to at-risk populations. He mentioned there is a lot of data they haven’t seen that would help the panel in their deliberations.
At the end they took a poll. How did they want the NIH and others to define that “high risk” category?
They cited healthcare workers and individuals at high risk due to occupational exposure to the virus. One FDA staffer mentioned teachers being included in the definition of “front line workers” at risk.
Dr. Kurilla said he wanted a better understanding of the true protection and the durability of the vaccine. “We just can’t simply be in a position where we would just be vaccinating people every time we think there’s a problem,” he said. “We really need to get a better handle on understanding exactly how these vaccines are mediating protection and the durability of that protection.”









Young people at extremely low risk of serious complications or death from a COVID infection need to be fully informed of the risks. The manufacturers, distributors, and investors behind these products have no incentive to reveal the potential adverse reactions, and some have a history of concealing the harms of medical products from young people (targeted to get the products), and their parents.
30-Year-Old Still Seeking Answers 6 Months After Developing Neurological Complications Following Pfizer Vaccine
‘I Just Want My Life Back’ Says 16-Year-Old Who Developed Neurological Symptoms After Pfizer Vaccine
Gates Earns 10X on BioNTech in Just Two Years: $55m Investment Now Over $550m
Pfizer received the largest criminal fine in history.
Bill Gates History of Vaccine Corruption Inflicting Harm and Death on Unsuspecting People in Poor Countries
“In the largest real-world observational study comparing natural immunity gained through previous SARS-CoV-2 infection to vaccine-induced immunity afforded by the Pfizer vaccine, people who recovered from COVID were much less likely than never-infected, vaccinated people to get Delta, develop symptoms or be hospitalized.”
Fully Vaccinated With Pfizer? You’re 6 to 13 Times More Likely to Get Delta Than Someone With Natural Immunity, Study Says
Apparently Moderns had 3x the quantity of spike protein which may increase immune response and duration of protection. Pfizer’s gda data showed 42% efficacy for delta which would have caused it to be rejected.
That 42% number was not mentioned in the hearing.
You’re right about the Moderna vaccine having 3 times the vaccine dose in each shot. That is more “potency” to trigger your immune response.
I believe Moderna also spaced out the length of time between shots, which “some” on the panel felt helped with it’s longer term protection.
So many cases of MyoCarditis and heart disorders after Pfizer vaccination in young people who were not duly warned of the risks. University mandates pushed healthy young people to get vaccinated who otherwise would not have. In WA state, Pfizer vaccines were injected into minors and young people at school vaccine clinics, public health clinics, and elsewhere with little to no warning about the risks of serious adverse events like blood clots, heart attacks and heart problems, strokes etc.
“Aiden Jo, 14, is spending his freshman year of high school sitting alone on the sidelines because he can’t engage in any physical activity, his mother said.
When most kids are in gym class, Aiden is resting in an effort to keep his heart rate lowered, after being diagnosed with heart inflammation doctors say he developed after receiving Pfizer’s COVID vaccine.
In an exclusive interview with The Defender, Emily Jo, Aiden’s mother, said before her son got the vaccine, she was led to believe his chance of suffering an adverse reaction was “one in a million.”
Childrens Health Defense reports, Nearly 15,000 Deaths, More Than 700,000 Injuries Reported to VAERS Since December 2020 Rollout of COVID Vaccines in U.S.Excerpts:
“In the U.S., 378.2 million COVID vaccine doses had been administered as of Sept. 10. This includes: 216 million doses of Pfizer, 148 million doses of Moderna and 15 million doses of Johnson & Johnson (J&J).
This week’s U.S. data for 12- to 17-year-olds show:
The most recent deaths involve one report of two patients [VAERS I.D. 1655100] who died after their second dose of Pfizer, including a 13-year-old female.
Other recent reported deaths include a 15-year-old boy (VAERS I.D. 1498080) who previously had COVID, was diagnosed with cardiomyopathy in May 2021 and died four days after receiving his second dose of Pfizer’s vaccine on June 18, when he collapsed on the soccer field and went into ventricular tachycardia; and a 13-year-old girl (VAERS I.D. 1505250) who died after suffering a heart condition after receiving her first dose of Pfizer.
Steve Kirsh is an entrepreneur not a medical doctor. This article gives way more airtime to the false testimony given than to the actual FDA responses.
Citing the government run VAERS is false testimony?
Interesting premise. Got any proof???
More interesting is ignoring the same questioning from the FDA panel. Was the panel’s line of questioning also false or wrong think?