On April 15 all citizens can sign up to get vaccinated; here’s a look at the options
We are now almost four months into vaccination distribution in Washington state, with counties now entering the Phase 3 guidelines for schools and businesses. Gov. Jay Inslee was in Vancouver last week touting the success of his administration’s COVID-19 programs. Over 3.7 million doses of the vaccines have been administered among the 7.6 million Washington citizens.
The Washington Department of Health (DOH) reports 346,470 confirmed cases of COVID-19, 24,232 possible cases, for a total of 370,652 cases as of April 6. That means fewer than five in 100 Washington citizens have contracted the virus. There have been 20,819 hospitalizations and 5,299 deaths. Less than 6 percent of people who get the virus end up in the hospital, although the state reports 44 percent of cases have an “unknown” status regarding hospitalization. The death rate is 1.4 percent of total cases.
According to the DOH, 30.7 percent of citizens have had at least their first vaccination, and 19.5 percent of people are fully vaccinated. Clark County has 26 percent with one vaccination and 16 percent or 78,219 people are fully vaccinated as of April 5.
There are three different vaccines available from the federal government; Pfizer-BioNtech, Moderna and Johnson & Johnson. All three are being administered under an “emergency use authorization” (EUA) by the Food and Drug Administration (FDA).
The Pfizer and Moderna vaccinations account for the majority of vaccinations administered so far. The Johnson and Johnson vaccine received FDA approval on Feb. 26, two months after Pfizer and Moderna.
Why Emergency Use Authorization?
The FDA allows for EUA “to facilitate the availability and use of medical countermeasures, including vaccines, during public health emergencies, such as the current COVID-19 pandemic. Under an EUA, FDA may allow the use of unapproved medical products, or unapproved uses of approved medical products in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions when certain statutory criteria have been met, including that there are no adequate, approved, and available alternatives.”
Normally, it takes 10 to 15 years for a new vaccine to be approved. Vaccine development is a long, complex process, involving a combination of government agencies and private firms. Studies to determine the best dose, dose schedule effectiveness, safety and side effects require long term follow-up.
Camas resident Brad Jensen, MD, is a pathologist with expertise in molecular diagnostics. During his pathology career, his area of specialty was microbiology. Jensen has served close to 30 years advising the state of Washington on laboratory issues. He is also a former lab medical director at PeaceHealth Southwest Medical Center and is board certified in Pathology and Internal Medicine.
“The COVID-19 vaccines were developed in less than a year,’’ Jensen said. “This is because all the US vaccines use new technologies that allow rapid development and production of vaccines. All you need is the RNA sequence of the virus. This allowed the studies to be started early in the pandemic. In addition, the new FDA director allowed the regulatory process to be fast-tracked at the request of the Trump administration. There was no compromise in safety for Americans.’’
This CDC link provides information on the three vaccines and was just updated on April 3.: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines.html
At the end of the 19th century, several vaccines for humans had been developed. They were smallpox, rabies, plague, cholera, and typhoid vaccines. Government regulation began in 1902.
The whole point of developing a vaccine is to trigger an appropriate immune response, where the body’s immune system recognizes a virus or pathogen, and quickly mounts an effective response.
One of the challenges with COVID-19 is that in many cases, the immune system goes into overdrive, triggering a cytokine storm which overwhelms the body. In a sense, it’s too much of a good thing that then causes harm.
Normal vaccine development
The first steps in vaccine development are normally laboratory and animal studies. This stage involves basic laboratory research and often lasts 2-4 years. Federally funded academic and governmental scientists identify natural or synthetic antigens that might help prevent or treat a disease. These antigens could include virus-like particles, weakened viruses or bacteria, weakened bacterial toxins, or other substances derived from pathogens.
Next, pre-clinical studies last 1-2 years, using tissue-culture or cell-culture systems and animal testing. They assess the safety of the candidate vaccine and its ability to provoke an immune response. Animal subjects may include mice and monkeys. These studies give researchers an idea of the cellular responses they might expect in humans. They may also suggest a safe starting dose for the next phase of research as well as a safe method of administering the vaccine.”
The next steps are three phases of clinical trials, normally lasting 6-7 years. They begin with a small group of 20-80 individuals. If successful, it moves to the next phase with larger groups, as they identify the vaccine’s safety, immunogenicity, proposed doses, schedule of immunizations, and method of delivery.
Phase III tests are randomized and double blind and involve the experimental vaccine being tested against a placebo. The goal is to assess vaccine safety in a large group of people. These studies also included tests to carefully study the body’s immune response to the virus.
The firm must then apply for FDA licensing and approval of their vaccine, with follow on monitoring of the vaccine after approval.
Are any of the three COVID-19 vaccines in the U.S. made from “virus-like particles, weakened viruses or bacteria, weakened bacterial toxins, or other substances derived from pathogens” cited above?
The COVID-19 vaccines are not the traditional type of vaccine.
Molecular-based vaccines
How do the US COVID-19 vaccines work? There are two types of COVID-19 vaccines — mRNA vaccines and a viral vector vaccine. Both involve stimulating an effective immune response against only a small non-disease-causing part of the entire virus The Pfizer and Moderna vaccines are mRNA, and the Johnson and Johnson product is a viral vector.
According to the Centers for Disease Control (CDC), two of the vaccines authorized by the FDA are messenger mRNA (mRNA) vaccines. In our bodies, mRNA is the template used to make proteins. mRNA vaccines teach your cells to produce a harmless piece of the coronavirus spike protein that then triggers an immune response to build antibodies (B-cells) and stimulate localized inflammatory cells (T-cells). In this way, you build immunity to COVID-19 without getting the illness. This mRNA is unable to make changes to our human DNA, according to Jensen.
Both mRNA vaccines are two dose vaccines. It typically takes about two weeks after the second dose to become fully protected. The vast majority of people vaccinated so far have received the mRNA vaccination.
According to a Live Science report, some of the most infamous viruses — HIV, the common cold virus, influenza and COVID-19 — put all their genetic information in RNA, with no DNA predecessor.
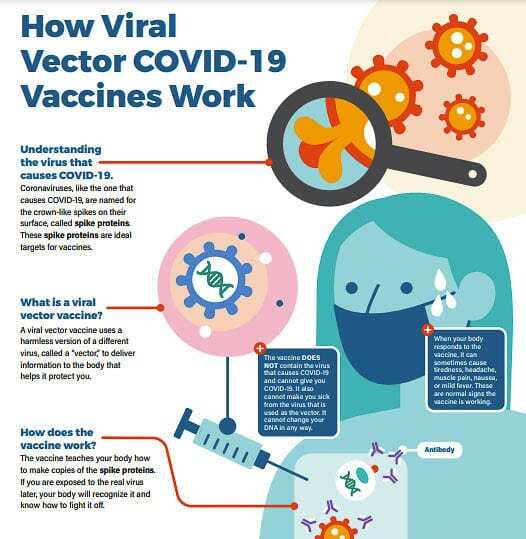
One of the COVID-19 vaccines authorized by the FDA (Johnson and Johnson) is a viral vector vaccine. Vector vaccines contain a weakened version of a virus — a different virus than the one that causes COVID-19 — that teaches your cells to make a harmless piece of coronavirus spike protein. This weakened virus cannot make new copies. Your immune system recognizes that the protein shouldn’t be there, which triggers your body to make antibodies that remember how to fight that virus if you are infected with COVID-19 in the future.
The CDC reports COVID-19 viral vector vaccines do not affect or interact with our DNA in any way. The genetic material delivered by the viral vector does not integrate into a person’s DNA.
The vector vaccine we have available is a one dose vaccine. It typically takes about two weeks after you get the vaccine to become fully protected.
“All three US vaccines have been shown in the Phase III studies to be 100 percent effective in preventing hospitalization or death,’’ Jensen said. “The commonly reported percentages of effectiveness describe only the effectiveness of preventing any symptoms of COVID-19. In addition, the popularly reported differences may be due to the numbers of active COVID-19 cases during the studies with the Johnson and Johnson study occurring during the fall wave of increased Covid-19 cases. These figures do not include results of testing performed during the study in the U.S.
In the last three decades, the number of known RNA varieties has blossomed, reports Live Science. Biologist Merlin Crossley wrote in The Conversation researchers discovered a menagerie of RNAs that do something completely different: regulate genes. “There’s a whole set of RNAs that play critical regulatory roles, influencing which genes get expressed and at what rates,” he said.
“In recent years, few areas of biology have been transformed as thoroughly as RNA molecular biology,” in large part due to the discovery of small regulatory RNAs, researchers wrote in a 2017 review published in the International Journal of Biomedical Science. Most significant are short interfering RNAs (siRNAs), microRNAs (miRNAs), and piwi-interacting RNAs (piRNAs), the authors wrote.
siRNAs and miRNAs “silence” genes by attaching to complementary sequences in mRNAs. The regulatory RNAs then activate complexes of proteins that can cut up the mRNA or block their translation, as described in a 2010 review published in the journal Current Genomics. siRNAs target invasive genetic material, like viral DNA, while miRNAs regulate an organism’s own genes, according to a 2009 review published in the journal Cell.
Doses administered
Bloomberg reports that the U.S. leads the world in total vaccines administered. Drugmakers have promised to deliver enough shots to fully vaccinate 130 million Americans by the end of March and 300 million people by the end of May. That would be more than enough for every adult. Clinical studies in children are presently underway.
Clark County Today asked the Washington DOH the following. “Can you tell us how many of each company’s vaccine has been administered to WA residents? Can you tell us how many of each has been administered to Clark County residents?
If a citizen has a preference, i.e. the ONE-SHOT Johnson & Johnson vaccine, can they get it? Or if they prefer the Pfizer vaccination, can they specify which one they want and how can they get the vaccine of their choice?
The DOH shared that for 16 and 17-year-olds, they are currently only authorized to receive the Pfizer vaccine, and provided the following information.
As of April 3, across the state a total of 3,722,703 doses of COVID-19 vaccine have been administered, including:
• Moderna: 1,713,301 doses
• Pfizer: 1,918,193 doses
• Johnson & Johnson: 89,966 doses
• Unknown: 1,243 doses
As of April 3, in Clark County a total of 180,527 doses of COVID-19 vaccine have been administered, including:
• Moderna: 37,973 doses
• Pfizer: 140,626 doses
• Johnson & Johnson: 1,884 doses
• Unknown: 44 doses
Doses administered are representative of what’s been allocated to the state from the federal government. Each vaccination site generally only gives one type of vaccine at a time. All three vaccines are proven to be safe and effective. Our advice is that people get the first vaccine that is offered to them, since they are all very effective at preventing severe disease and death. (The exception is 16 and 17-year-olds who are currently only approved for Pfizer).
In its Thu., April 1 briefing, the DOH expressed concern about a “4th wave” of the virus, saying we are still in the midst of the pandemic. “We’re not out of the pandemic yet,” said Dr. Umar Shaw, Secretary of Health. The state has opened up eligibility for vaccinations to all citizens, 16 and older, on April 15. That will be the 4-month anniversary of the vaccine rollout in the state. DOH mentioned that 5 million Washingtonians are presently eligible to get the vaccine.
Dr. Shaw emphasized that citizens should take whatever vaccine is available to them. Here in Clark County, the overwhelming majority of vaccines available and administered have been the Pfizer vaccine.
The DOH vaccine finder website does not presently contain the ability for people to search for locations to obtain a specific brand of vaccine. They hope to update the site soon, so people can find a specific type, especially for parents of 16 and 17 year old children needing the Pfizer vaccine.
The DOH vaccine finder website is one of numerous websites people can use to schedule vaccination.
This Vaccinefiner website supposedly allows people to search by zip code and by each of the three approved vaccines.




