Inexpensive drug appears to shorten hospital stays, possibly a use as prophylactic treatment
In the battle against COVID-19, the Delta variant is currently getting the most attention, as roughly three out of four cases being reported by health officials are this variant of the coronavirus. “Breakthrough cases” are being reported as 125,000 vaccinated individuals have now gotten this version of COVID.
People are understandably seeking numerous ways to prevent getting the virus. Boosting your immune system is important and low vitamin D levels appear to significantly increase people’s risk of getting the virus.
What about Ivermectin or other alternative “repurposed” medications?
Last fall, local Physicians Assistant Scott Miller shared an early perspective on helping his patients with possible medications. Ivermectin is one that he’s seen a decrease in viral replication within 48 hours. It’s anti-parasitic, but it can be vital for especially at-risk patients, and it’s very affordable, Miller said.
In December, Dr. Pierre Kory testified in Congress about the positive impact Ivermectin had in fighting the virus. He represented the Front-Line COVID-19 Critical Care Alliance (FLCCC). They were interested in finding research on the use of any other already existing, safe, low-cost therapeutic agents.
“Ivermectin is highly safe, widely available, and low cost,” Kory said. “Its discovery was awarded the Nobel Prize in medicine, and is already included on the WHO’s ‘World’s List of Essential Medicines.’
CLICK TO PLAY VIDEO. Dr. Pierre Kory of the Front-Line COVID-19 Critical Care Alliance addresses a Senate committee and answers questions about the use and effectiveness of ivermectin.
“We now have data from over 20 well-designed clinical studies, ten of them randomized, controlled trials, with every study consistently reporting large magnitude and statistically significant benefits in decreasing transmission rates, shortening recovery times, decreasing hospitalizations, or large reductions in deaths.”
The National Institutes of Health said in February there was insufficient data to “recommend either for or against the use of ivermectin for the treatment of COVID-19.” Lab tests found the drug stopped the reproduction of the SARS-CoV-2 virus that caused the disease. However, to be effective, the dosages would need to be “100-fold higher than those approved for use in humans.”
In March, the Food and Drug Administration (FDA) issued a special warning that “you should not use ivermectin to treat or prevent COVID-19. Using any treatment for COVID-19 that’s not approved or authorized by the FDA, unless part of a clinical trial, can cause serious harm,” the FDA says.
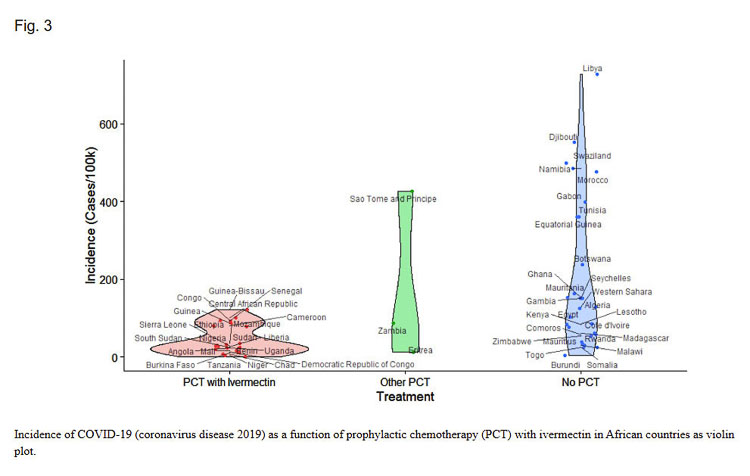
A study first published in Nov. 2020 reported a lower incidence associated with prophylactic administration of ivermectin by evaluating countries in Africa that regularly take the drug for parasitic infections.
“We show that countries with routine mass drug administration of prophylactic chemotherapy including ivermectin have a significantly lower incidence of COVID-19. Prophylactic use of ivermectin against parasitic infections is most common in Africa and we hence show that the reported correlation is highly significant both when compared among African nations as well as in a worldwide context.”
The authors noted that the percentage of the overall population that received prophylactic treatment using ivermectin mostly ranged from 30–90 percent, yet “there was no significant difference in the resulting incidence of COVID-19. Even the lower treatment coverages achieved the same reductions resulting from MDA reaching nearly the entire population.” The reasons for this fact are so far unexplained.
The Jerusalem Post reported Aug. 2 that a new Israeli study shows positive results. A small randomized, controlled, double-blind trial from May 2020, through January 2021, evaluated the effectiveness of ivermectin in reducing viral shedding among non-hospitalized patients with mild to moderate COVID-19.
Nearly 72 percent of volunteers treated with ivermectin tested negative for the virus by day six. In contrast, only 50 percent of those who received the placebo tested negative.
The study also looked at how infectious the patients were, and found that only 13 percent of ivermectin patients were infectious after six days, compared with 50 percent of the placebo group – almost four times as many.
The study did not prove ivermectin was effective as a prophylactic, meaning that it could prevent disease, nor did it show that it reduces the chances of hospitalization.
The American Journal of Therapeutics did release a study showing some positive results for prophylactic use of ivermectin, in addition to its use in treating COVID-19.
Their conclusion states: “Moderate-certainty evidence finds that large reductions in COVID-19 deaths are possible using ivermectin. Using ivermectin early in the clinical course may reduce numbers progressing to severe disease. The apparent safety and low cost suggest that ivermectin is likely to have a significant impact on the SARS-CoV-2 pandemic globally.”
Yet the World Health Organization (WHO) has recommended against using the drug except in clinical trials. The Israeli doctor in the above referenced story said he was very disappointed that the WHO did not support any trial to determine whether the drug could be viable.
“Developing new medications can take years; therefore, identifying existing drugs that can be repurposed against COVID-19 [and] that already have an established safety profile through decades of use could play a critical role in suppressing or even ending the SARS-CoV-2 pandemic,” wrote the researchers in the American Journal of Therapeutics. “Using repurposed medications may be especially important because it could take months, possibly years, for much of the world’s population to get vaccinated, particularly among low- to middle-income populations.”
Evaluating “repurposed drugs” is what Dr. Kory and his FLCCC Alliance are all about.
“Why is the FDA attacking a safe, effective drug” asked David Henderson and Charles Hooper in the Wall St. Journal last month.
“Ivermectin is on the World Health Organization’s List of Essential Medicines. Merck has donated four billion doses to prevent river blindness and other diseases in Africa and other places where parasites are common. A group of 10 doctors who call themselves the Front Line Covid-19 Critical Care Alliance have said ivermectin is “one of the safest, low cost, and widely available drugs in the history of medicine.”
They report 70 clinical trials are evaluating the use of ivermectin for treating COVID-19. The statistically significant evidence suggests that it is safe and works for both treating and preventing the disease.
Fewer ivermectin patients developed respiratory distress (2.6 percent vs. 15.8 percent); fewer required oxygen (9.6 percent vs. 45.9 percent); fewer required antibiotics (15.7 percent vs. 60.2 percent); and fewer entered intensive care (0.1 percent vs. 8.3 percent). Ivermectin-treated patients tested negative faster, in four days instead of 15, and stayed in the hospital nine days on average instead of 15. Ivermectin patients experienced 13.3 percent mortality compared with 24.5 percent in the control Group.
Moreover, the drug can help prevent Covid-19. One 2020 article in Biochemical and Biophysical Research Communications looked at what happened after the drug was given to family members of confirmed Covid-19 patients. Less than 8 percent became infected, versus 58.4 percent of those untreated. Among 200 healthcare workers and others at high risk of exposure, only 2 percent of those given ivermectin developed Covid-19. But 10 percent of the control group did.”
They cite the American Journal of Therapeutics. “Out of four billion doses administered since 1998, there have been only 28 cases of serious neurological adverse events.” The same study found that ivermectin has been used safely in pregnant women, children and infants

Dr. Kory and FLCCC Alliance reported in May that South Africa, Zimbabwe, Slovakia, Czech Republic, Mexico, and India, have approved the drug for use by medical professionals.
In June, Oxford University in the UK added ivermectin to a major study.They indicate little data from large-scale randomised controlled clinical trials are available to validate its effect on recovery speed or decreasing hospitalisation. They hope to change that.
Chief investigator professor Chris Butler said: “Ivermectin is readily available globally, has been in wide use for many other infectious conditions so it’s a well-known medicine with a good safety profile, and because of the early promising results in some studies it is already being widely used to treat COVID-19 in several countries.
“By including ivermectin in a large-scale trial, we hope to generate robust evidence to determine how effective the treatment is against COVID-19, and whether there are benefits or harms associated with its use.”
The Oxford University Principle Trial has more than 5,000 participants and will give a three-day course of oral ivermectin treatment to individuals randomly and compare their results to individuals who will receive standard care.
In the US, the NIH is evaluating therapeutics for COVID-19 with its Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) master protocol. Ivermectin was added in phase three of ACTIV-6, which will test the effectiveness of repurposed drugs.
There has been a growing number of people taking ivermectin for animals as word spread on social media about its possible use to cure COVID-19. This has resulted in some people calling state poison centers after taking the incorrect dosage since the medication is intended for animals.
“If the FDA were driven by science and evidence, it would give an emergency-use authorization for ivermectin for Covid-19,” said Henderson and Hooper. “Instead, the FDA asserts without evidence that ivermectin is dangerous.
“At the bottom of the FDA’s warning against ivermectin is this statement: ‘Meanwhile, effective ways to limit the spread of COVID-19 continue to be to wear your mask, stay at least 6 feet from others who don’t live with you, wash hands frequently, and avoid crowds.’ Is this based on the kinds of double-blind studies that the FDA requires for drug approvals? No.”
Also read:
Boosting your immune system to stay healthy in era of Delta variant of COVID-19

Opinion: We should be questioning the global suppression of early treatment options for COVID-19










